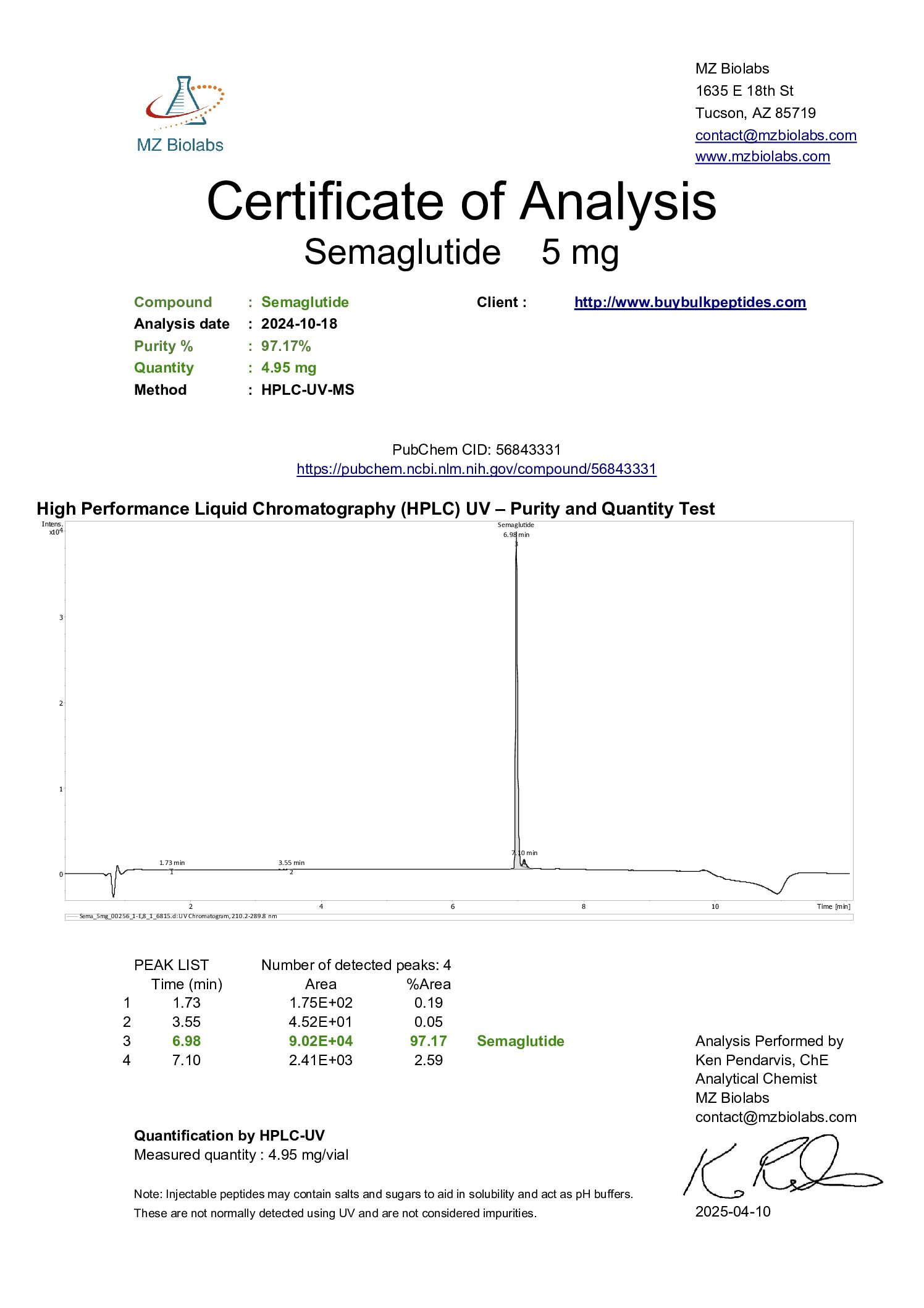
In the field of weight management therapies, an emerging drug is Semaglutide peptide. It is a GLP-1 receptor agonist originally approved for treating type 2 diabetes (T2D). Semaglutide weight loss therapy has recently made headlines.
With intriguing evidence pointing to significant weight loss potential, researchers are focusing on the potential of Semaglutide. As a GLP-1 receptor agonist, Semaglutide functions in a unique way. It affects appetite regulation & energy balance.
This article explores Semaglutide weight loss therapy, its emerging role, and its potential impact on the field.
So if you are interested in researching Semaglutide, this guide offers detailed insights into this GLP-1 receptor agonist. Let’s start our journey.
What is Semaglutide Peptide?
Semaglutide is an innovative and promising treatment that operates as a glucagon-like peptide-1 (GLP-1) receptor agonist. It belongs to the GLP-1 family, renowned for stimulating insulin and decreasing glucagon secretion. Semaglutide shares a 94% homology to human GLP-1. So it ensures a reduced risk of immunogenicity. (Kalra et al., 2020)
Semaglutide: Chemical Structure and Unique Characteristics
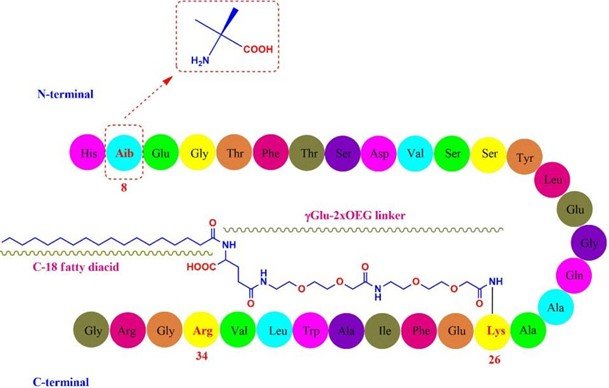
Chemically, Semaglutide presents as a peptide composed of 31 amino acids. This peptidic structure features certain unique attributes. Here’s an overview of these attributes:
- Semaglutide has an alanine residue at the 8th position, which is substituted with 2-aminoisobutyric acid. This particular substitution safeguards semaglutide from degradation by the DPP-4
- The peptide also contains an arginine substitution at the 34th Originally a lysine residue in native GLP-1 is present here. This change facilitates the production of GLP-1 analogs through a semi-recombinant process.
- Finally, a lysine residue at the 26th position has been acylated to allow for the attachment of a C18 fatty A hydrophilic linker, “γGlu-2xOEG”, is used for this attachment. This addition elongates the systemic half-life through enhanced albumin binding and reduced renal clearance. (Al Musaimi et al. 2018)
Enhanced Stability and Prolonged Half-Life
Semaglutide is a GLP-1 receptor agonist that has gained attention for its effectiveness in treating obesity and managing type 2 diabetes. A key factor contributing to its success is its enhanced stability and prolonged half-life, which differentiates it from other medications in the same class.
Structural Modifications for Enhanced Stability:
- C-terminal modification: The addition of a modified amino acid at the C-terminus of the peptide chain enhances the stability of semaglutide by protecting it from degradation by enzymes in the body.
- Peptide engineering: The overall design of the peptide sequence is optimized to minimize interactions with enzymes and other factors that could lead to degradation.
Prolonged Half-Life:
- Reduced clearance: The structural modifications also reduce the clearance of semaglutide from the body, resulting in a longer half-life.
- Delayed absorption: Semaglutide is formulated as an injection, and the specific formulation can be designed to delay the absorption of the medication into the bloodstream. This can further prolong its half-life.
- Reduced dosing frequency: Due to its prolonged half-life, semaglutide can be administered less frequently, typically once a week. This improves patient convenience and adherence.
Now let’s discuss the Semaglutide mechanism of action and its effects.
Semaglutide Weight Loss Mechanism of Action & Effects
Semaglutide exerts its effects by activating GLP-1 receptors. Glucagon-like peptide-1 is a major incretin hormone in humans. It is recognized for its multiple roles in metabolism and glucose regulation.
Through activating these receptors, semaglutide augments insulin secretion in a glucose-dependent manner. It also inhibits glucagon release and suppresses hepatic
gluconeogenesis. On top of that, GLP-1 receptor activation by semaglutide helps delay gastric emptying. Ultimately, Semaglutide weight loss occurs by reducing appetite and energy intake.
Let’s take a detailed look at the effects of Semaglutide.
Semaglutide’s Effects on Various Body Systems
Semaglutide impacts multiple body systems:
- Pancreas: By stimulating the GLP-1 receptors in the pancreas, semaglutide enhances insulin secretion. This aids in maintaining proper blood glucose balance.
- Gastrointestinal Tract: Semaglutide slows gastric emptying. It leads to a feeling of fullness and a reduction in overall food intake.
- Fat Tissue: It is understood that GLP-1 receptor agonists can also influence fat tissue by reducing lipolysis. It can contribute to overall weight loss.
- Central Nervous System: Semaglutide interacts with GLP-1 receptors in the brain, particularly in appetite & reward-related regions. Activation of these receptors leads to decreased responses to food stimuli.. (Mahapatra et al. 2022)
Out of all these effects, here’s a very significant outcome of Semaglutide.
Semaglutide, Appetite Suppression, and Satiety Induction
One of the significant outcomes of semaglutide’s action in the body is its effect on appetite and satiety.
The delayed gastric emptying caused by semaglutide leads to an extended feeling of fullness. Furthermore, stimulating GLP-1 receptors in the brain influences the neural response to food. It leads to reduced cravings and decreased food intake.
Evidence of semaglutide’s role in appetite suppression & satiety induction was observed in a study. It was a randomised, crossover, placebo-controlled trial. The study involved obese type 2 diabetes patients and normoglycemic obese and lean individuals.
The trial found that obese subjects and patients with type 2 diabetes showed:
- Increased brain responses to food pictures in appetite and reward-related brain (Ard et al. 2021)
The Result: Semaglutide Weight Loss
By acting in the manner described above, semaglutide effectively induces weight loss. The combined effects of:
- Insulin regulation,
- Decreased glucagon release,
- Slowed gastric emptying,
- Appetite suppression,
contribute to reduced body weight. Moreover, the unique design of semaglutide enables it to resist proteolytic degradation. It allows Semaglutide to function longer in the body and provides sustained benefits for individuals managing their weight.
Now let’s look at some clinical studies on Semaglutide weight loss therapy.
Semaglutide Weight Loss Clinical Studies
Numerous clinical trials have explored its efficacy and safety. They revealed significant potential in facilitating weight loss and improving metabolic health. This body of research presents compelling evidence for using semaglutide in the fight against the global obesity epidemic.
The following sections will delve into the details of four pivotal studies. These studies shed light on the multifaceted benefits of semaglutide.
Study 1: Wider Benefits of Semaglutide in Obesity from the STEP Program
This study evaluated the impact of semaglutide on quality of life, control of eating, and body composition in adults with obesity. The results of the study showed that:
- Semaglutide significantly improved all IWQOL-Lite-CT scores related to physical function and psychosocial
- Improvements in all Control of Eating questionnaire domains were noted up to week There were significant differences in craving control and craving for savoury lasting until week 104.
- The total fat mass reduction was greater with semaglutide than with (O’Neil et al., 2022)
Study 2: Weekly Semaglutide Use in Adults with Overweight or Obesity
This trial examined the effects of semaglutide on body weight, cardiometabolic risk factors, physical functioning, and adverse events. The results are:
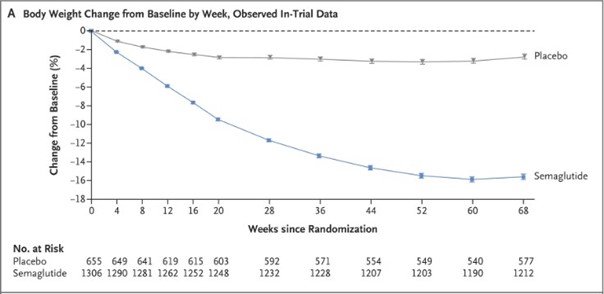
- Participants on semaglutide had an average weight loss of 9% at week 68 compared to a 2.4% loss in the placebo group.
- More participants in the semaglutide group achieved weight reductions of 5% or more (86.4% vs 5%), 10% or more (69.1% vs 12.0%), and 15% or more (50.5% vs 4.9%).
- Improvements were seen in cardiometabolic risk factors and physical functioning in the semaglutide
- Nausea and diarrhea were common but generally mild and transient. Gastrointestinal events led to more treatment discontinuations in the semaglutide group (4.5% vs 8%). (Wilding et al. 2021)
Study 3: Semaglutide’s Impact on Energy Intake, Appetite, and Gastric Emptying
This study investigated semaglutide’s effects on energy intake, appetite, control of eating, gastric emptying, and safety. Here are the results:
- Semaglutide reduced energy intake by 35% compared to
- Significant reductions in hunger and prospective food The increases in fullness and satiety were also reported in the semaglutide group.
- Better control of eating and fewer/less intense food cravings were reported with
- No evidence of delayed gastric emptying was The safety was consistent with the known profile of semaglutide. (Friedrichsen et al. 2021)
Study 4: Semaglutide as a GLP-1 Receptor Agonist for Type 2 Diabetes Management
This review focused on semaglutide’s development, clinical studies, pharmacotherapy, and more.
- Semaglutide demonstrated strong anti-hyperglycemic activity in multiple clinical trials involving adults, elderly & obese type 2 diabetic
- Despite gastrointestinal side effects, semaglutide was well-tolerated. It provided better glycemic control with a low risk of hypoglycemia and good patient
- The SUSTAIN-6 and PIONEER-6 studies confirmed the cardiovascular safety of semaglutide for long-term (Mahapatra et al. 2022)
Now let’s move on to the dosage and administration of Semaglutide.
Semaglutide Dosage & Administration
Semaglutide is typically administered in a controlled & gradually escalating dosage. It maximises its weight loss benefits and minimises potential side effects. This stepwise approach begins at a low dose and progressively increases over time. The recommended starting dose is:
- 25mg weekly for the first four weeks.
- The dosage is then increased to 5mg in weeks 5-8.
- Increased to 1mg in weeks 9-12.
- Then 7mg is given in weeks 13-16.
- From week 17 and onwards, the full dose of 4mg per week is administered.
This medication is administered once a week via subcutaneous injection. The duration of treatment in clinical studies ranges from 52 to 104 weeks. It depends on the objectives and parameters of the study. It’s also critical to note that the dosage should not exceed 2.4mg per week.
Adherence to this dosing protocol is crucial for the optimal effectiveness of semaglutide. In the event of a missed dose, it can be taken within five days. However, the dose should be skipped entirely if this window is missed. (Chao et al., 2022) (Alabduljabbar et al. 2022)
After looking at the dosage, here are some effects shown by a study.
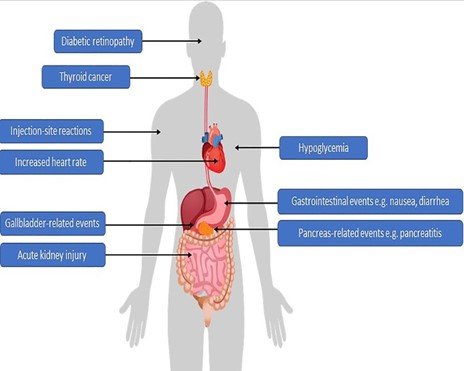
Common Side Effects and Adverse Events
Semaglutide, while generally well-tolerated, can cause some side effects. These are usually mild and often resolve over time. However, some people may experience more severe adverse events.
Common Side Effects:
- Gastrointestinal: Nausea, vomiting, diarrhea, constipation, abdominal pain, and flatulence.
- Injection-related: Pain, redness, or swelling at the injection site.
- Other: Headache, fatigue, and dizziness.
It’s important to note that these are not all possible side effects. If you experience any unusual or concerning symptoms while taking semaglutide, it’s crucial to consult with your healthcare provider.
Essential Precautions for Semaglutide
Semaglutide has gained recognition as an effective treatment for obesity. However, like any medication, it comes with specific safety considerations to ensure that patients use it safely and effectively.
Monitor for Side Effects
- Watch for Gastrointestinal Symptoms: Common side effects include nausea, vomiting, diarrhea, and constipation. Monitoring these symptoms, especially during the initial phase of treatment, can help manage them effectively.
- Report Severe Reactions: If you experience severe abdominal pain, persistent nausea, vomiting, or signs of an allergic reaction (such as swelling or difficulty breathing), seek immediate medical attention.
Who Should or Should Not Take Semaglutide for Weight Loss
Ideal Candidates.
- Individuals with obesity: Semaglutide is for BMI 27+ with a weight-related condition like diabetes or hypertension.
- People struggling to lose weight: If you’ve tried traditional diet and exercise methods without success, semaglutide may be a viable option.
- Individuals with type 2 diabetes: Semaglutide can help manage blood sugar levels and promote weight loss in people with type 2 diabetes.
Not Recommended for.
- Children and adolescents: Semaglutide is not approved for use in individuals under the age of 18.
- Pregnancy and breastfeeding: Pregnant or breastfeeding women should avoid semaglutide.
- Individuals with certain medical conditions: People with specific health conditions, such as pancreatitis or a history of thyroid tumors, may not be suitable candidates.
Where to Buy Semaglutide Peptide?
For those seeking to buy Semaglutide online for research purposes, Bulk Peptide Supply offers a reliable and high-quality service.
- Helpful Customer Support: Your questions will be answered promptly by friendly team, ensuring a smooth shopping experience.
- Quality Guarantee: All products are meticulously tested in our labs to meet stringent purity standards, guaranteeing the reliability of your research.
The Bulk Peptide Supply community comprises many researchers and scientists. They rely on the company’s quality, precision, and expertise for their critical projects. By choosing Bulk Peptide Supply, you align with a trusted ally in advancing scientific understanding.

Semaglutide Peptide from Bulk Peptide Supply
Conclusion
Semaglutide peptide weight loss therapy showcases exciting potential. Its effectiveness in managing chronic overweight and obesity conditions makes it a promising candidate for further research. We must stay informed and prepared as we anticipate new developments in Semaglutide weight loss therapy. For researchers aiming to explore the potential of Semaglutide further, Bulk Peptide Supply offers a reliable, high-quality source.
References
Al Musaimi, O., Al Shaer, D., de la Torre, B. G., & Albericio, F. (2018). 2017 FDA peptide harvest. Pharmaceuticals (Basel, Switzerland), 11(2), 42. https://doi.org/10.3390/ph11020042
Alabduljabbar, K., Al-Najim, W., & Le Roux, C. W. (2022). The Impact Once-Weekly Semaglutide 2.4 mg Will Have on Clinical Practice: A Focus on the STEP Trials.
Nutrients, 14(11), 2217. https://doi.org/10.3390/nu14112217
Ard, J., Fitch, A., Fruh, S., & Herman, L. (2021). Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Advances in Therapy, 38(6), 2821–2839. https://doi.org/10.1007/s12325-021-01710-0
Chao, A. M., Tronieri, J. S., Amaro, A., & Wadden, T. A. (2022). Clinical insight on semaglutide for chronic weight management in adults: Patient selection and special considerations. Drug Design, Development and Therapy, Volume 16, 4449–4461. https://doi.org/10.2147/dddt.s365416
Friedrichsen, M., Breitschaft, A., Tadayon, S., Wizert, A., & Skovgaard, D. (2021). The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes, Obesity & Metabolism, 23(3), 754–762. https://doi.org/10.1111/dom.14280
Kalra, S., & Sahay, R. (2020). A review on semaglutide: An oral glucagon-like peptide 1 receptor agonist in managing type 2 diabetes mellitus. Diabetes Therapy : Research, Treatment and Education of Diabetes and Related Disorders, 11(9), 1965–1982. https://doi.org/10.1007/s13300-020-00894-y
Mahapatra, M. K., Karuppasamy, M., & Sahoo, B. M. (2022). Semaglutide is a glucagon-like peptide-1 receptor agonist with cardiovascular benefits for managing type 2 diabetes. Reviews in Endocrine & Metabolic Disorders, 23(3), 521–539. https://doi.org/10.1007/s11154-021-09699-1
O’Neil, P. M., & Rubino, D. M. (2022). Exploring the wider benefits of semaglutide treatment in obesity: Insight from the STEP program. Postgraduate Medicine, 134(sup1), 28–36. https://doi.org/10.1080/00325481.2022.2150006
Smits, M. M., & Van Raalte, D. H. (2021). Safety of Semaglutide. Frontiers in Endocrinology, 12, 645563. https://doi.org/10.3389/fendo.2021.645563
Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M. T. D., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., & Kushner, R. F. (2021). Once-Weekly semaglutide in Adults with overweight or obesity. New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/nejmoa2032183
Disclaimer
All products provided by NuScience Peptides are intended for research purposes only. The discussions provided in this blog are purely for education & research purposes. The US FDA (Food & Drug Association) has not approved the drug or any information contained here.
Any potential applications of Semaglutide peptide must be carried out in a controlled research setting, adhering to all relevant ethical and regulatory guidelines. Use of Semaglutide outside of this context is not recommended nor endorsed by NuScience Peptides.